Joseph A. Henske, MD, and Michelle L. Griffith, MD, are fellows, and Michael J. Fowler, MD, is an assistant professor of medicine in the Division of Diabetes, Endocrinology, and Metabolism in the Department of Medicine at Vanderbilt University Medical Center in Nashville, Tenn. Dr. Fowler is an associate editor of Clinical Diabetes.
Clin Diabetes 2009;27(2):72–76Joseph A. Henske , Michelle L. Griffith , Michael J. Fowler; Initiating and Titrating Insulin in Patients With Type 2 Diabetes. Clin Diabetes 1 April 2009; 27 (2): 72–76. https://doi.org/10.2337/diaclin.27.2.72
Download citation file:
toolbar searchA 48-year-old African-American man presents to his primary care physician for follow-up of his type 2 diabetes. His diabetes is complicated by peripheral neuropathy, although he has no evidence of retinopathy or nephropathy. His medical history also includes obesity, hyperlipidemia, hypertension, and obstructive sleep apnea.
At a visit 3 months earlier, his A1C was 9.2% on a regimen of glipizide extended release, 10 mg daily, and metformin sustained release, 1,000 mg twice daily. He and his physician discussed the possible need for insulin, but he preferred to try an additional oral agent first. Sitagliptin, 100 mg daily, was added to his previous regimen, and he was continued on his angiotensin receptor blocker and statin therapies.
At this visit, he reports that he tolerated the sitagliptin poorly because of diarrhea and stopped taking it after only a few weeks. His A1C today is up to 9.4%. His weight is 151 kg, with a BMI of 43 kg/m 2 . His physical exam is significant for the presence of obesity and acanthosis nigricans. After a thorough discussion of the morbidity associated with poorly controlled diabetes, he agrees to initiate insulin therapy.
The initiation of insulin is an important stage in the management of type 2 diabetes. Like the patient in this case, many patients with diabetes are unable to achieve a goal A1C on oral therapies alone. Given the progressive nature of this disease, characterized by gradual impairment in β-cell function and loss of β-cell mass, 1 most patients with type 2 diabetes will eventually require insulin therapy to achieve a goal A1C of < 7% as defined by the American Diabetes Association (ADA) or ≤ 6.5% as defined by the American Association of Clinical Endocrinologists.
In 1993, the Diabetes Control and Complications Trial confirmed the long-suspected notion that tight glycemic control was crucial to prevent the microvascular complications of type 1 diabetes. 2 Evidence for this conclusion was strengthened by the U.K. Prospective Diabetes Study, first published in 1995, which showed that a similar reduction in complications could be achieved by tightly controlling blood glucose in patients with type 2 diabetes. 3-5 Intensive glycemic control has also been shown to reduce risk of cardiovascular disease in patients with type 1 diabetes, though this has not been demonstrated in patients with type 2 diabetes. 6-8
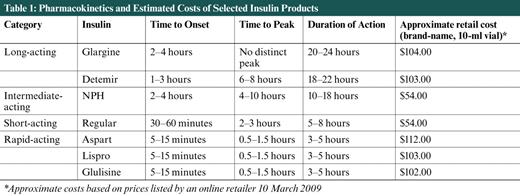
Pharmacokinetics and Estimated Costs of Selected Insulin Products


Despite these findings, recent evidence suggests that too often providers delay in transitioning from oral agents to insulin. Based on one recent study, the A1C level that triggers glucose-lowering action is > 9%. 9 In this article, we discuss an approach to initiation and titration of insulin in patients in the primary care setting.
A major challenge for primary care physicians when initiating insulin therapy is choosing when to use each of the many insulins available today—rapid-acting, short-acting, intermediate-acting, long-acting, or premixed insulins (Table 1). To use insulin therapy most effectively, the regimen must be matched to the individual patient, considering his or her lifestyle needs and physical and mental capabilities, in addition to matching the body's physiological requirements.
Insulin is produced by the β-cells of the pancreas to clear glucose from the blood and allow uptake of glucose by the muscle, fat, and liver. The pancreas makes a small amount of insulin, termed “basal insulin,” even in the fasting state to suppress catabolism of muscle, fat, and other body tissues and regulate hepatic glucose production. The β-cells must also respond to the glucose challenge of carbohydrate consumption and secrete insulin in appropriate amounts after a meal in an exquisitely regulated manner to maintain normal glucose levels. Insulin must be precisely released to avoid both postprandial hyperglycemia from insufficient or delayed insulin release or hypoglycemia from secretion of too much insulin. These are the physiological mechanisms that physicians attempt to replicate with exogenous insulin therapy.
Many algorithms have been generated for initiating insulin therapy, and this can be confusing to general practitioners. One clinical pearl is that, although it is of utmost importance to achieve glycemic control in the long term, this does not have to be done in hours or days. In fact, rapid improvement in glycemic control can actually be associated with adverse outcomes in terms of bleeding from proliferative retinopathy, 10 patient dissatisfaction, or even danger related to frequent hypoglycemia. Therefore, the classic mantra, “Start low, and go slow” holds true here.
Rather than starting with multiple daily injections of different types of insulin, often it is more manageable for patients and physicians to begin with a once-daily basal insulin instead of a rapid-acting insulin to be taken before meals for nutrient coverage. Recently published studies have compared the effects of adding basal versus prandial coverage in patients with type 2 diabetes on oral agents. The Treating to Target in Type 2 Diabetes study group found that, although prandial insulin improved A1C slightly more than basal insulin in patients with type 2 diabetes on maximal doses of metformin and sulfonylurea, there was more frequent hypoglycemia with the prandial insulin. 11
Introducing insulin with one or two shots daily of a long- or intermediate-acting insulin to provide basal coverage may be an easier transition to make and is an accepted approach in the ADA's clinical guidelines. 12 Available long-acting agents include once-daily insulin glargine and once- or twice-daily insulin detemir. Twice-daily NPH, an intermediate-acting insulin, is also an option for basal coverage, although this may be associated with more frequent hypoglycemia than detemir 13 (Table 1).
A third alternative to initiating basal versus bolus insulin therapy is the use of biphasic premixed insulin preparations. These may offer the advantage of providing basal and postprandial coverage in one injection. The 1-2-3 Study demonstrated that initiation of once-daily predinner biphasic insulin aspart 70/30 allowed 41% of patients with a mean baseline A1C of 8.6% who were not at goal on two or more oral therapies or one oral therapy and basal insulin to achieve the ADA-recommended target A1C of < 7%. 14 Using an aggressive titration protocol involving 12 patient-provider interactions every 16 weeks, this insulin dose was rapidly increased. A second injection was added before breakfast and a third before lunch if pre- and postprandial goals were not achieved to eventually allow 70-77% of patients to achieve goal A1C on two or three injections daily. 14 Despite demonstration of success with this regimen, many endocrinologists choose not to use fixed-dose insulin preparations because of the perceived increased ability to better tailor a patient's insulin dosages to his or her individual needs by separating the basal and bolus insulins.
Deciding which basal insulin to choose is up to the provider and the patient. Glargine does not have a distinct peak, has onset of action within 2-4 hours, and a duration of action of 20-24 hours. It can be given every 24 hours at any time of day convenient for the patient. 15 The onset of detemir's action is 1-3 hours after injection, its peak occurs in 6-8 hours, and its duration of action is 18-22 hours. 15 Given this variability in duration, some patients may be able to use this insulin just once per day, whereas others will require twice-daily injections. Compared to these two basal insulins, NPH insulin has the advantage of lower cost, but it must be used twice or three times per day for true basal coverage. Some providers may use it at bedtime only to provide control of fasting blood glucose overnight in patients using other agents during the day. It is an intermediate-acting product, with onset of activity in 2-4 hours, a peak in 4-10 hours, and a duration of 10-18 hours. 15
Two accepted approaches for choosing a dose of basal insulin include starting with a fixed dose of 10 units per day or determining a weight-based dose of 0.2 units/kg of body weight (in patients with usual insulin sensitivity and renal and hepatic function/day). 12 For the patient in the case above, 0.2 units/kg would suggest a starting dose of ~ 30 units. Given the patient's weight and his significantly elevated A1C of 9.4%, this is a perfectly acceptable starting dose. Therefore, this patient could receive 30 units of insulin glargine injected subcutaneously once daily; if NPH or twice-daily detemir were chosen, the total starting basal dose would be divided into two doses of 15 units each, with injections spaced ~ 12 hours apart. Another approach to the use of NPH insulin is to give two-thirds of the total dose in the morning and one-third in the evening; this allows the peak of NPH to provide some prandial coverage at midday.
Although many clinicians recommend continuing all oral agents, one should be attuned to the risk of hypoglycemia with concomitant insulin and sulfonylurea therapy. Metformin may be continued throughout insulin therapy given that its mechanism of action to suppress hepatic glucose production is distinct from that of insulin. Patients should monitor and record fasting blood glucose levels every morning and should monitor more frequently (3-4 times per day) to watch for hypoglycemia and document excursions in blood glucose to help guide further adjustments in therapy. Every 3 days, if the fasting blood glucose is not in the target range of 70-130 mg/dl, the dose of basal insulin can be increased by 2 units if glucose is relatively close to the fasting target (e.g. if fasting blood glucose is 130-180 mg/dl), or 4 units if fasting blood glucose is > 180 mg/dl after 3 days of monitoring. 12 If hypoglycemia with blood glucose < 70 mg/dl occurs, basal insulin should be decreased by 10% or 4 units, whichever yields the larger change. 12 Should severe hypoglycemia occur, more drastic reduction in dosing may be warranted.
A common pitfall with basal insulin dosing is increasing the dose too much before adding prandial insulin. Often, providers may note continual “fasting” hyperglycemia and titrate glargine up to 60, 80, or 100 units per day before adding prandial insulin. In these cases, the basal insulin is actually treating the postprandial hyperglycemia. Patients are never truly in a fasting state, and blood glucose never decreases appropriately after meals. Unfortunately, because of the relative ease of doing so, providers and patients often elect to increase the dose of the daily basal insulin instead of adding mealtime injections. This does not match the physiological needs, however, and predisposes patients to fasting hypoglycemia. A rule of thumb is that a patient should not be advanced to more than 0.5 units/kg of body weight for basal insulin without first considering adding a rapid-acting insulin (e.g., 0.1 units/kg) with meals.
If A1C remains elevated ≥ 7% after 2-3 months on basal insulin, or if prelunch, predinner, or bedtime blood glucose levels are clearly above the goal of 70-130 mg/dl despite a fasting glucose level at goal, prandial therapy should be instituted. Although some oral agents may be useful as adjuncts to basal insulin and metformin in this setting, we will focus on the addition of prandial insulin.
Insulins available for prandial coverage include regular insulin (defined as short-acting) and the rapid-acting insulin analogs (Table 1). The rapid-acting analogs, including aspart, lispro, and glulisine, allow a closer approximation of physiological insulin secretion. 15 They are absorbed more rapidly than regular insulin, leading to a more rapid onset (5-15 minutes) and peak (about 30-90 minutes) and a shorter duration of action (3-5 hours). 15 Their rapid onset allows them to be given just before meals, and they should not be given more than 15 minutes before meals. Regular insulin is less expensive than the analogs. Its onset of action occurs in 30-60 minutes, requiring dosing 30 minutes before meals for best effect. Regular insulin peaks at 2-3 hours and has a duration of 5-8 hours. 15
When adding or adjusting prandial insulin, it is absolutely crucial that patients understand the importance of frequent blood glucose monitoring. Patients on basal and bolus insulin therapy should monitor blood glucose no less than four times daily (at meals and bedtime), including before any carbohydrate intake. By studying patterns of blood glucose levels prelunch, predinner, and at bedtime, one can make appropriate adjustments to add insulin to the preceding mealtime to prevent hyperglycemia at the next blood glucose reading.
Although individual insulin requirements vary widely, we will propose a plan to initiate prandial insulin that can be titrated over time. Although one may choose to add insulin to be given before each meal, this may not be necessary for all patients. If prelunch blood glucose is consistently elevated > 130 mg/dl, add rapid-acting insulin at breakfast. If predinner blood glucose is elevated, add rapid-acting insulin at lunch. If bedtime blood glucose is elevated, add rapid-acting insulin at dinner. For each of these doses, one can often begin with 4 units (in patients with usual insulin sensitivity and renal and hepatic function) and adjust by 2 units every 3 days until blood glucose is in range.
This is an effective method with the caveat that patients consume a consistent amount of carbohydrate at each meal. For patients who have difficulty with this or would like more flexibility with their diet, one should consider developing a personalized insulin-to-carbohydrate (I:C) ratio. This requires patients to count the number of carbohydrate consumed at each meal and give a variable amount of insulin according to the determined ratio. For example, patients with an I:C ratio of 1:10 would take 1 unit of insulin for every 10 grams of carbohydrate consumed.
This can also be adjusted according to acceptability of blood glucose readings. If readings are consistently elevated, the ratio can be decreased to 1:8 or 1:5. If readings are too low prelunch, predinner, or at bedtime, the ratio could be increased to 1:12, 1:15, or 1:20 as indicated. This method usually requires motivated patients and at least one session with a registered dietitian or diabetes nurse educator to teach carbohydrate counting, but it can be very effective in controlling blood glucose, offer increased flexibility in meal content, and limit the possibility of hypoglycemia after a low-carbohydrate meal.
Occasionally, insulin therapy can be complicated by unexpected hypoglycemia or hypoglycemia unawareness. This is a particularly challenging situation for patients and providers. Options for these patients include insulin pumps and continuous glucose monitoring (CGM) devices. Insulin pumps allow patients to vary their basal rate on an hourly basis, decreasing the rate overnight or with exercise, or increasing it to account for insulin resistance caused by early morning secretion of cortisol and growth hormone. CGM devices can be worn temporarily under a physician's direction to better detect patterns in blood glucose variation or permanently, such as for people with type 1 diabetes and severe episodes of hypoglycemia.
Despite some advantages over insulin injections, both of these devices require a high degree of patient motivation and are not typically used by patients with type 2 diabetes unless they are already very comfortable with frequent blood glucose monitoring, carbohydrate counting, and basal and bolus insulin administration and still demonstrate need for improved glycemic control. Meta-analysis of continuous subcutaneous insulin infusion (CSII) versus multiple daily injection (MDI) therapy in patients with type 1 or type 2 diabetes showed that CSII leads to better glycemic control, lower insulin requirements, and no increase in hypoglycemia for those with type 1 diabetes, but the same is not true for those with type 2 diabetes. 16 CSII does not result in better glycemic control versus MDI therapy in type 2 diabetes, and there is no difference in insulin requirements or hypoglycemic events. 16
Before adjusting patients' medication regimen, physicians must have a thoughtful discussion with them regarding the benefits and potential risks of the new medication. This is especially true when initiating insulin therapy, which comes with technical and psychological barriers that must be overcome for the treatment to be used effectively. Often, patients have misperceptions about insulin therapy that can be easily cleared up with a few moments of explanation or demonstration. Demonstration of the use of a prefilled, disposable pen device by a physician or nurse at the time insulin use is discussed can be very effective in allaying patient fears about injection pain, inconvenience, and technical difficulty.
Insulin initiation can sometimes cause patients to feel a sense of guilt or that they are somehow being punished for poor control of their diabetes. It is important for physicians to understand these feelings and reassure patients that the need for insulin therapy is because of the progressive nature of diabetes and is intended solely to make patients feel better and reduce their risk of complications.
A 2006 meta-analysis by Ali et al. 17 determined that the prevalence of comorbid depression in patients with type 2 diabetes is 17.6%, suggesting that one in six patients with diabetes suffers from depression. Depression leads to adverse outcomes in diabetes and can lead to poor compliance with insulin therapy; thus, patients should be screened to identify and treat psychological issues that may affect care. 18
In conclusion, initiation of insulin therapy is an important stage in management of patients with type 2 diabetes. In accordance with ADA guidelines, 12 insulin should be added either as the first agent when clinically indicated or when A1C is not at goal on one to two oral hypoglycemic agents. Physicians, patients, and health care teams should carefully consider and overcome any psychological barriers to initiation and work closely together to prescribe a physiological regimen to control fasting and postprandial blood glucose levels.